

A conventional procedure cannot calculate it because it is a qualitative feature.Įlectronegativity measures the tendency of an atom to attract and establish bonds with electrons. Major periodic trends are listed as follows:Įlectronegativity is a chemical property that describes the ability of an atom to attract bond pairs of electrons. The unknown properties of any element can be deduced in parts due to these periodic trends. These trends exist due to the periodic nature of the elements and their identical atomic structure within their various group families. Periodic trends and chemical reactivity provide chemists with a valuable tool for swiftly predicting an element’s attributes. These periodic trends in chemical and physical properties occur due to the periodic table’s arrangement. In other words, the elements with similar electronic configurations present similar properties. This periodicity is caused due to recurrence of similar electronic configurations throughout the periodic table.
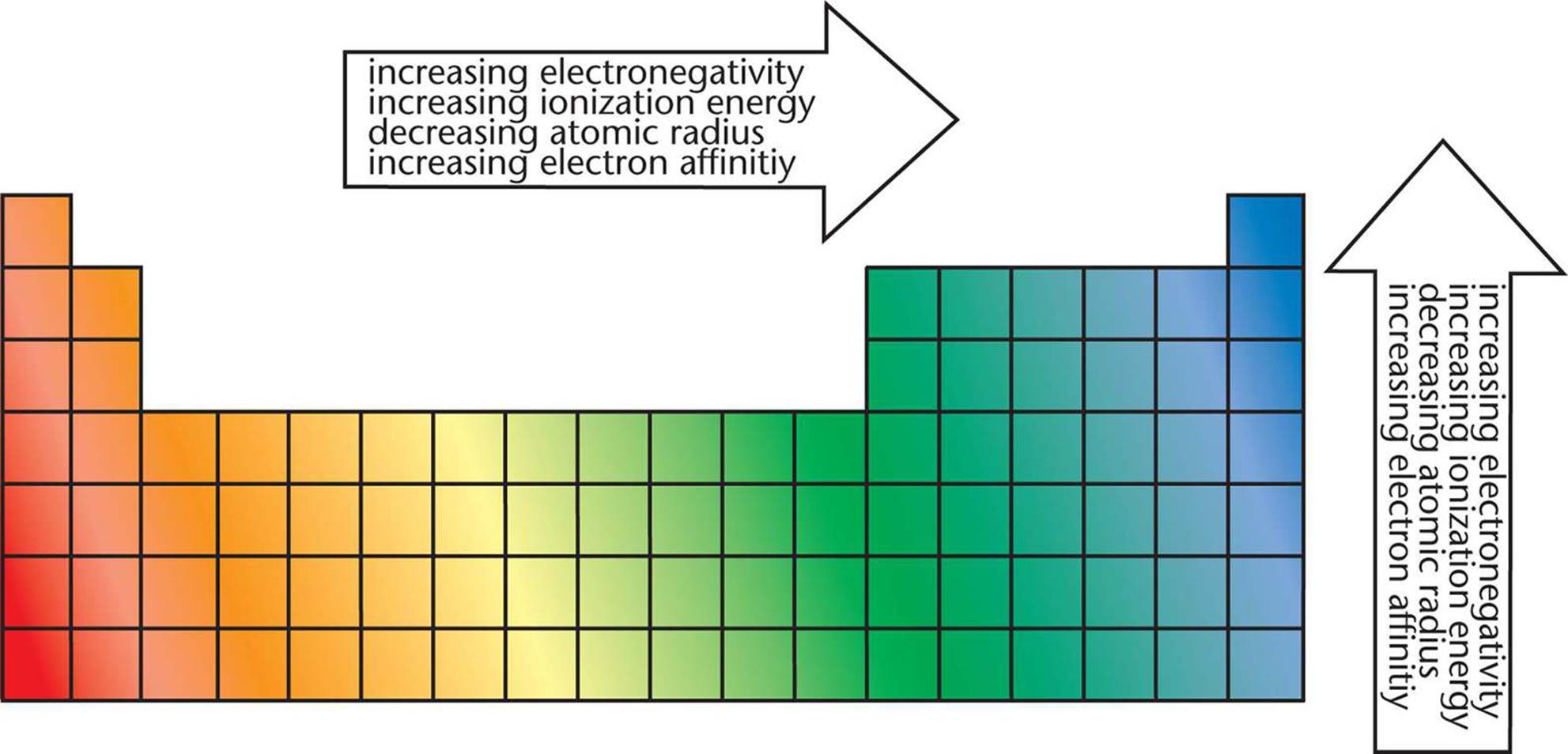
Periodic trend is a unique pattern in the periodic table that presents different aspects such as specific element’s size and electrical properties. What is a Periodic Trend in Chemical Properties? For instance, atomic size decreases from left to right in the table however, certain exceptions that do not follow such periodic table trends are also observed.
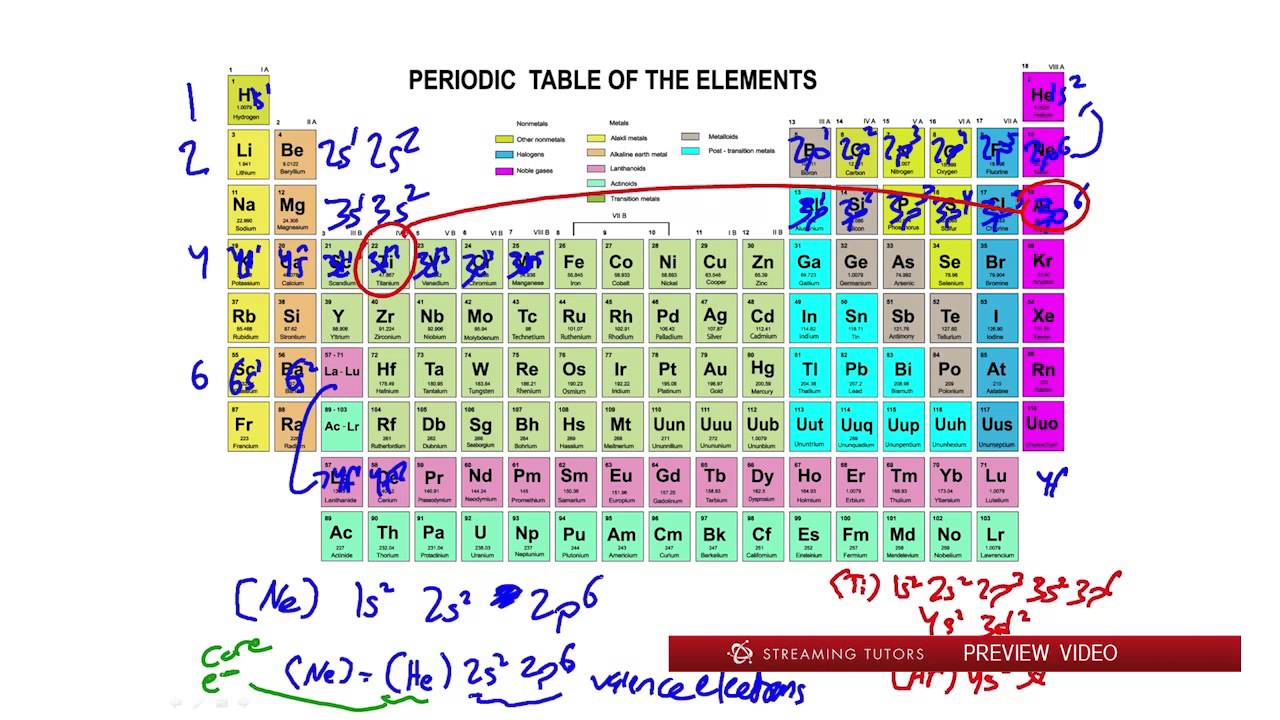
Thus, the elements display periodicity in their properties. According to the modern periodic table, elements are arranged based on their atomic numbers, which are directly linked to the physical and chemical properties of the elements. It states that the chemical and physical properties of the elements form the periodic functions of their atomic numbers.


 0 kommentar(er)
0 kommentar(er)
